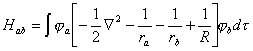|
|
|||||||||||||||||||||
|
|
||||||||||||||||||||||
| ЕБЧАЮЛжУ ->Ёь5ЁЁЖрдзгЗжзгвЛ -> Р§ЬтгыЯАЬт
-> СЗЯАЬт(2/2) |
|
Р§ЬтгыЯАЬт ЖўЁЂСЗЯАЬт(2/2)(ЫФ)ЃЎЮЪД№ЬтЃК 1ЃЎЬжТлЯТСаЗжзгЕФЙЙаЭЃК (1)XeF4 (2)XeO4 (3)XeO3 (4)XeOF2 (5)XeOF4ЁЃ(ВЮПМД№АИЃЉ НтЃК(1)ЦНУце§ЗНаЮЃЛ 2ЃЎЪдБШНЯЯТСаЗжзгжаC-OМќМќГЄЃЌВЂЫЕУїРэгЩЃК (a)CO2 (b)CO (c)CH3COCH3ЁЃ(ВЮПМД№АИЃЉ НтЃКC-OМќГЄДЮађЃКCH3COCH3>CO2>CO 3ЃЎЪдгУЧАЯпЙьЕРРэТлЬжТлЯТУцЕФЗДгІЪЧЗёПЩвдНјааЃК НтЃКC2ЕФHOMOЃЈ1
4ЃЎЪдгУЧАЯпЙьЕРРэТлНтЪЭЃКЮЊЪВУДввЯЉМгЧтЕФЗДгІБиаыдкДпЛЏМСДцдкЯТВХФмНјааЃП НтЃКвђЮоДпЛЏМСЪБЖдГЦВЛЦЅХфЃЛгУ NiзїДпЛЏМСНЋЮќИН H2БфГЩ HдзгеМгаЕчзгЕФЙьЕРЃЌКЭввЯЉЕФ LUMOЖдГЦЦЅХфЁЃ 5ЃЎЪдЗжЮіЯТСаЗжзгжаЕФГЩМќЧщПіЃЌжИГіC-ClМќМќГЄДѓаЁДЮађВЂЫЕУїРэгЩЁЃ (a)CH3Cl (b)H2C=CHCl (c)CHЁдCClЁЃ(ВЮПМД№АИЃЉ НтЃК H3CClжа CЮЊ sp3дгЛЏЃЌ C-ClЮЊ ІвЕЅМќЁЃ H2C=CHClжа CЮЊ sp2дгЛЏЃЌ C-ClМфГ§ЕЅМќЭтЛЙгаДѓ ІаМќ
6ЃЎЗжЮіH2+ЕФНЛЛЛЛ§ЗжЃЈІТЛ§ЗжЃЉHabЮЊИКжЕЕФИљОнЁЃ(ВЮПМД№АИЃЉ НтЃК
ЁЁЁЁЁЁ ЁЁЁЁвђ EH=-13.6eV, SabЮЊе§жЕЃЌЙЪЕквЛЯюЮЊИКжЕЃЛдкЗжзгЕФКЫМфОрЬѕМўЯТЃЌ KЮЊИКжЕЁЃЫљвд HabЮЊИКжЕ 7ЃЎЫЕУїH2+ЕФМќГЄБШH2ГЄЃЌЖјO2+ЕФМќГЄБШO2ЖЬЕФдвђЁЃ(ВЮПМД№АИЃЉ НтЃК H2+БШ H2дкГЩМќЙьЕР (Ів1s)ЩЯЩйвЛИіЕчзгЃЌ H2+ЕФМќМЖЮЊ 0.5ЃЌ H2ЕФМќМЖЮЊ 1ЁЃ Ѓ Ѓ O2+БШO2дкЗДМќЙьЕР(Іа2p*)ЩЯЩйвЛИіЕчзгЃЌO2+ЕФМќМЖЮЊ2.5ЃЛO2ЕФМќМЖЮЊ2.0 8ЃЎЯжга4sЃЌ4pxЃЌ4pyЃЌ4pzЃЌ3dz2, 3dx2-y2, 3dxy, 3dxz, 3dyzЕШОХИідзгЙьЕР,ШєЙцЖЈzжсЮЊМќжсЗНЯђ,дђЫќУЧжЎМф(АќРЈздЩэ)ПЩФмзщГЩФФаЉЗжзгЙьЕРЃПИїЪЧКЮжжЗжзгЙьЕРЃП(ВЮПМД№АИЃЉ НтЃК ІвЙьЕР :
s-s, s-pz, s-dz2,ЁЁ
pz-pz,
pz-dz2, dz2- dz2, Ѓ Ѓ Ѓ ІаЙьЕРЃК px-pxЃЌ px-dxyЃЌ py-pyЃЌ py-dxzЃЌ dyz-dyzЃЌ dxz-dxz Ѓ Ѓ Ѓ ІФЙьЕРЃК dxy-dxyЃЌ dx2-y2-dx2-y2 9ЃЎаДГіЗжзгO2, C2ЕФЗжзгЙьЕРЕФЕчзгзщЬЌ(ЛљЬЌ)ЃЌВЂжИГіЫќУЧЕФДХадЁЃ(ВЮПМД№АИЃЉ НтЃК O2
: [KK(Ів2s)2(Ів*2s)2(Ів2px)2(Іа2px)2(Іа2py)2(Іа*2px)1(Іа*2py)1] 10ЃЎОйР§ЫЕУїЪВУДЪЧІвЙьЕРЁЂІаЙьЕРКЭІФЙьЕРЁЃ(ВЮПМД№АИЃЉ НтЃК ЩшМќжсЗНЯђЮЊ zжсЃЌдзгЙьЕР sгы s, sгы pz, pzгы pzзщКЯ (ЭЗЖдЭЗ )ЃЌЕУЕНЕФЗжзгЙьЕРЪЧдВжљЖдГЦЕФЃЌГЦЮЊ ІвЙьЕРЃЛдзгЙьЕР pxгы px, pyгы pyзщКЯЃЈМчВЂМчЃЉЃЌЕУЕНЕФЗжзгЙьЕРЭЈЙ§МќжсгавЛИіНкУцЃЌГЦЮЊ ІаЙьЕРЃЛдзгЙьЕР dx2-y2гы dx2-y2, dxyгы dxyзщКЯЃЌЕУЕНЕФЗжзгЙьЕРЭЈЙ§МќжсгаСНИіНкУцЃЌГЦЮЊ ІФЙьЕРЃЛ 11ЃЎаДГіCOЕФЕчзгзщЬЌМАЛљЬЌЦзЯюЁЃ НтЃК 1Ів22Ів23Ів21Іа45Ів2
ЃЌ 1ІВ 12ЃЎЪдгУЗжзгЙьЕРРэТлЬжТлOHЛљЕФНсЙЙЁЃ ЃЈ1ЃЉаДГіOHЛљЕФЕчзгзщЬЌВЂЛГіФмМЖЭМ ЃЈ2ЃЉЪВУДРраЭЕФЗжзгЙьЕРЛсгаЮДГЩЖдЕчзг ЃЈ3ЃЉЬжТлДЫЙьЕРЕФаджЪ ЃЈ4ЃЉБШНЯOHЛљКЭOH-ЛљЕФзюЕЭЕчзгдОЧЈЕФФмСПДѓаЁ НтЃК (1)OHЛљЕФЕчзгНсЙЙЮЊЃК
(1Ів)2(2Ів)2(3Ів)2(1Іа2px)2(1Іа2py)1
Ѓ Ѓ Ѓ (2)ЮДГЩЖдЕчзгеМОн ІаЙьЕР Ѓ Ѓ Ѓ (3)1ІаЙьЕРЪЧЗЧМќЙьЕРЃЌШдБЃГж OдзгЕФ 2pЙьЕРЕФЬиад Ѓ Ѓ Ѓ (4)OH-ЕФзюЕЭЕФЕчзгдОЧЈЕФФмСПБШ OHЛљЕФвЊИпЁЃ
Ѓ Ѓ ЁЁЃЈ2ЃЉБШНЯCO2ЃЌCOКЭБћЭЊжаCЃOМќМќГЄЫГађЃЌВЂЫЕУїРэгЩ НтЃК (1)kk1Ів22Ів21Іа43Ів2
Ѓ Ѓ Ѓ Ѓ (2)БћЭЊжазюГЄЃЌ COЦфДЮЃЌ CO2жаЕФ C-OМќГЄзюЖЬЁЃвђ CO2жага 2Иі
Ѓ Ѓ (3)
Cr(CO)6 ЁЁе§АЫУцЬх ЁЁЁЁЁЁЃ Fe(CO)6
ЁЁШ§НЧЫЋзЖ |